We’ve got great news for our medical products consumers: MOKO‘s medical dust-free workshop has been EN ISO 13485:2016 certified by TUV SUD. We now bear the globally-recognized trademark of trust and quality to ensure that our clients get safe, credible, secure, and sustainable medical devices to render dependable solutions.

What’s ISO 13485 Certified
ISO13485 is the ultimate QMS (Quality Management System) standard to vet the eligibility of medical devices. Its main aim is to ensure medical products and related services are of the best quality to function optimally. Any company or organization that designs, develops, produces, services, stores, distributes or installs medical assets needs to meet these regulatory standards before deploying its services to consumers. As always, MOKO puts our customers’ interests first and have met these standards.
This certification is recognized worldwide. ISO13485 is currently the FDA’s mandatory Quality Management System after the U.S proposed an arrangement that harmonized ISO 1385:2016 with U.S. FDA 21 CFR 820. The ISO13485 Certified can be given to any registered organization, provided it meets the regulatory requirements no matter its size. However, the certification cannot be issued to one person since it’s not a personal standard.
Since the ISO13485 is not a set standard for products, it does not dictate medical products’ quality. Instead, it’s a product-based standard to be followed by targeted organizations.
Quality Management System for Medical Devices
Medical devices Quality Management System (QMS) is an organized system comprising standard requirements for procedures and processes involved in the design, prototyping, manufacturing, supply, and storage of medical devices. Since ISO doesn’t accredit or certify any company for complying with its requirements, it mandates other organizations like TUV SUD to do the accreditation. Therefore, the ISO 13485, which is the globally-recognized QMS for the medical device industry, just outlines the safety and qualification standards required for a company to get the ISO 13485 certification from TUV SUD.
Thus, for any company to get the TUV SUD certification, it must manifest its capacity to provide medical devices and solutions that constantly meet its clients’ and necessary regulatory requirements.
The new edition of ISO 13485 certification— ISO 13485:2016 builds upon the previous ISO 9001 by adding more regulatory requirements. This new version was established on the 1st of March, 2016, and brings to the mix of technological advancements and global requirements evolution of up to 12 years.
EN ISO 13485 vs. ISO 13485 Certification
Most people conflict with the terms EN and ISO and are sometimes caught at the crossroad between the two, wondering which standards to adopt or comply with. In order to differentiate EN ISO 13485 from ISO 13485, we need to understand what EN standards and ISO standards mean.
EN standards are also known as the European harmonised standards. Their purpose is to confirm compliance with the vital set regulatory requirements of the European CE injunctions. This means that pre-existing ISO safety standards must be incorporated in the EN safety standard without being altered.
On the other hand, ISO safety standards are the set regulatory requirements crafted by the standardization institute—ISO to be used by a third-party organization like TUV SUD to verify a company’s compliance in order to be certified.
In general, EN standards are not independent standards created by an EN organization but are often IEC or ISO safety standards harmonised by the European Commission (EC).
For instance, the ISO can create a standard, let’s say the ISO 45000 family — Occupational health and safety. Then, if a European Union member country adopts the standard, the EC must publish it as an EN standard since it’s harmonised. When this happens, all other members of the EU will then have to approve and adopt this harmonised standard and get rid of any contradicting state standards. The ISO standard has to be renamed with the prefix EN upon harmonization. For instance, EN ISO 13485.
Now that the hard part is out of the way, we can now differentiate EN ISO 13485 from ISO 13485 Certification. EN ISO 13485 certification is offered by an organization mandated to approve that a company meets the harmonised ISO 13485 standards in the EU region. In contrast, the ISO 13485 Certification is awarded to companies that have complied with the ISO 13485 standards.
ISO13485 Certification Process
Every organization dealing with medical devices must go through strict requirements. This is to make sure they fully comprehend the process needed to make and offer medical devices. MOKO was no exception. We underwent a thorough audit by a CB (Certified body) before we got our ISO 13485 certification, expected to run for three years. After three years, we’ll undergo another audit to get re-certified.
These requirements are updated regularly hence the need for re-certification after a period of time.
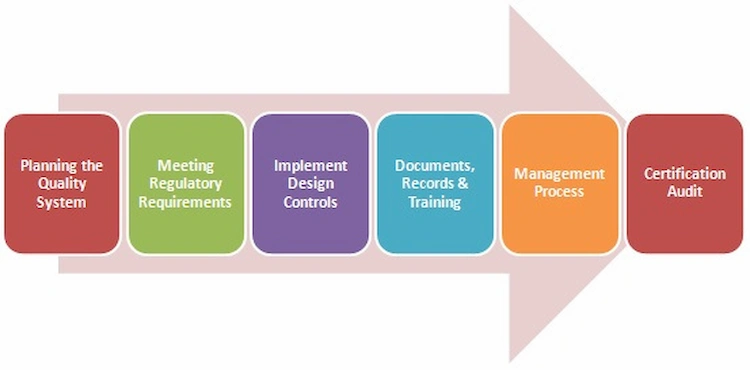
The certification process is a 6-steps journey. First, the organization needs to document and present its quality plants to implement changes in the QMS. Second, they must meet the regulatory requirement as per the region they are selling their products to. Third, they must implement design protocol as per their client’s needs. The Fourth step requires organizations to present their documentation for records and training in their quality management process. On the fifth step, the Certified Board evaluates the risk management process for the organization, which leads to the last step of the certification audit. This is done by special personnel from the medical sector and an ISO agent. Once the audit is complete, several corrective plans might be suggested, which the organization is supposed to accept so as to be issued the final ISO13485 certificate.
Clauses in the ISO 13485 Standard
1. (Clause 4) Responsibility QMS: Dictates the general requirements and the documentation requirements for the company that needs certification to meet.
2. (Clause 5) Management Responsibilities: Outlines the Quality policy, management review, planning, responsibility, communication & authority, management commitment, and customer focus.
3. (Clause 6) Resource management: Outlines the infrastructure, human resources, the work environment & contamination control plan, and source/provision of resources.
4. (Clause 7) Product Realization: Outlines the production & service provision, control of monitoring & measuring tools, the risk-based approach towards product achievement, design & development, and customer-related processes.
5. (Clause 8) Processes for product Measurement, Analysis, and Improvement: Outlines the monitoring & measurement, the analysis of data, the control of non-conforming products, and the product’s improvement.
How to Select the Correct Safety Standards
To ensure you don’t get kicked out of the market due to failure to get certified by ISO or EN, you need to follow these steps. They will help you meet the set safety, environmental, and health standards.
1. Answer the following: Type of product, Target market, Target audience/customer, Country to sell your merchandise, Latest ISO/EN/IEC standards to refer to, State/local legislation applying to your merchandise.
2. Choose the right standard: Is there any State/Local EN or ISO or IEC standard that applies to your product? Comply with all codes of practice set in the region you’re trading in.
3. Leverage a digital tool to find the relevant standards to comply with based on the location and other keywords like the type of product.
Advantages of Getting ISO 13485 Certified
Here’s why you should get your ISO 13485 medical device and related products certified:
1. It enhances your processes and procedures’ quality, effectiveness, and transparency. This will increase trust with your clients and your brand’s credibility.
2. It confirms the quality, reliability, efficiency in performance, and safety of your medical devices.
3. Boosts your reputation and consumer’s satisfaction
4. Works as a marketing tool for your company
5. There are no sale returns since your products will have met the standard quality and safety measures enough to impress and satisfy your clients.
It’s no secret that a medical device can strike the balance the life or death of a consumer. With that in mind, MOKO always strives to pass the quality management audit in the design, development and production processes to ensure the final product improves our consumers’ lives. This is why we’ve been ISO13485 certified over and over again.
Other than that, we’ve become UL, RoHS, ISO 14001 and ISO I9001 in an effort to better our overall operations for our customers.